This is an old revision of the document!
Table of Contents
oSpim
oSPIM stands for Oblique SPIM. The light sheet is generated using an objective below the sample (an oil objective) and is tilted partway towards TIRF not unlike HiLo illumination. The imaging objective is a water dipping objective that is lowered into the media orthogonal to the light sheet. The oSPIM is an ideal tool for cell biologists or other applications where the sample is in close contact with the coverslip. The same concept was independently and concurrently developed by a research group in Heidelberg and called the πSPIM (see their publication). For more details about oSPIM see ASI's presentation given in December 2016 at ASCB.
Micro-Manager plugin
There is a Micro-Manager plugin for the oSPIM, based heavily on the diSPIM plugin but with a few changes. In the future hopefully the oSPIM vs. diSPIM will hopefully be a setting changed by the user in the plugin, but for now there is a flag in the source code that needs to be changed and the code be rebuilt.
Start by installing a recent nightly build of Micro-Manager 1.4.x. The latest official build is quite old at this point, so make sure to grab a nightly build. For Windows the nightly builds are here.
Download the latest copy of the plugin here (last update 22-Jun-2017) or else one from the archives. Make sure Micro-Manager is not running and then copy the JAR file into C:\Program Files\Micro-Manager-1.4\mmplugins (it will take precedence over the ASIdiSPIM plugin in the Device_Control folder, or you can delete ASIdiSPIM.jar from that folder and replace it with this one).
Assembly
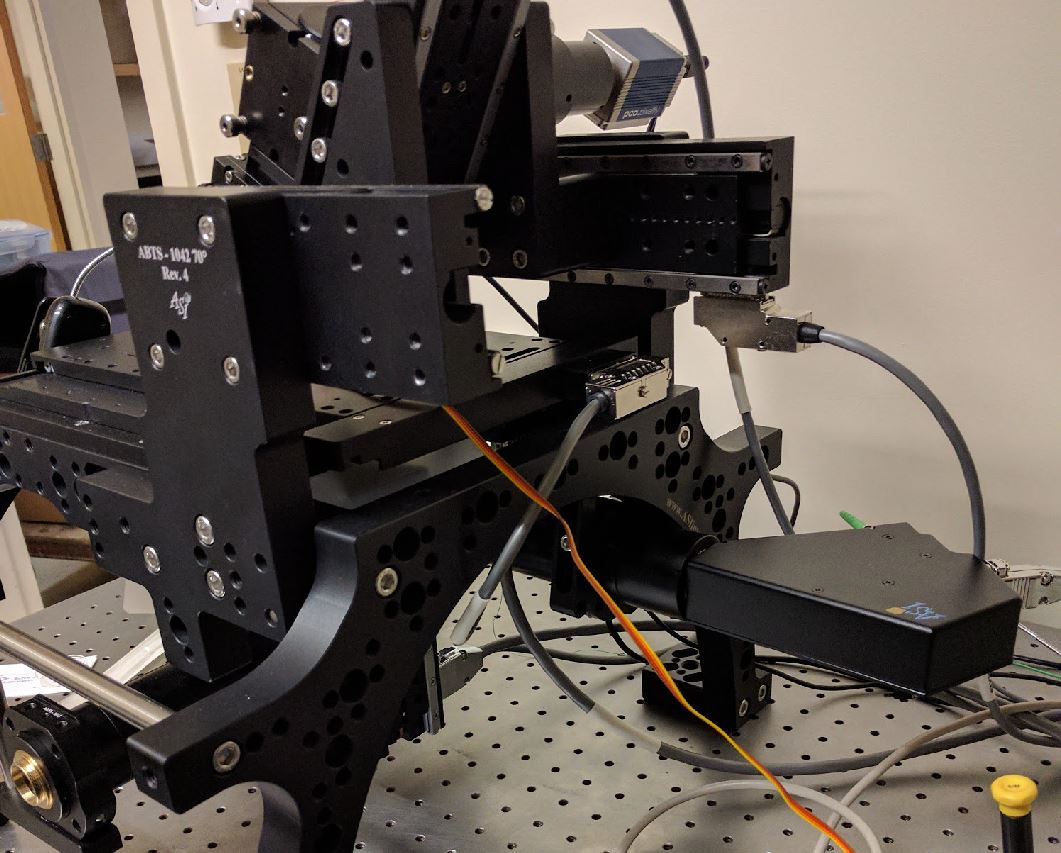
Much like the diSPIM. “F” axis in the semi-vertical SPIM head, “G” is the horizontal SPIM head axis, and “Z” is the vertical inverted microscope motorized axis. The camera trigger goes to PLC output #1 and the laser TTLs go to PLC outputs #5-8 just like diSPIM.
There is a Cube III body in the SPIM head, inside put one D-cube after mounting a 25x36mm ultra-flat mirror (not a dichroic, but installed in the usual dichroic position) as well as an emission filter. The Cube III body just below the objective in the inverted microscope gets a dichroic (to reflect the excitation beam but transmit the emission) as well as an emission filter.
The scanner should be rotated with the fiber optic connector towards the back, as shown here:
The SPIM camera should be rotated so that it is rotated the same as the cube and other elements in the SPIM head.
Alignment
Alignment of the oSPIM is significantly easier than the diSPIM though many of the same principles apply. This documentation is a work in progress so don't hesitate to ask your system integrator if you need more information.
Coarse Light Sheet Alignment
Start by getting things aligned “to eye”, then move on to the fine alignment using the microscope cameras.
Make sure you are getting the beam out of the bottom objective. Add a sample, e.g. dish with fluorescent dye, and steer the bottom dichroic on the kinematic adjusters until the beam is coming out at approximately the correct angle, orthogonal to the top objective (not straight up, not tilted to TIRF, but somewhere in between tilted the correct direction). If you cannot achieve that (or the kinematic adjusters is extremely tilted) then need to check the optical path before the dichroic. There is an offset ring installed between the scanner tube lens and the bottom mirror to intentionally move the beam off-center so that the dichroic tilt doesn't have to do much work (the beam should hit on the right side of the objective BFP so that it tilts to the left exiting the objective). You can remove the objective and put a witness target there if you need to mess with the optical path before the dichroic.
Inverted Microscope Alignment
You should have received some pieces of white translucent silicone with a hole in the middle. These are splash guards to prevent any spilled liquid from entering the inverted microscope. The hole should form a tight seal when placed over the objective, if it's loose get the next smallest hole.
Move the bottom objective until it's focused on the coverslip surface using transillumination (probably room lights are enough) as viewed with the camera on the inverted microscope. A dirty coverslip helps, or put a dot of marker on the top (sample) surface of a coverslip and look for that.
Once the light sheet is aligned (see steps below) then you can adjust the tilt of the mirror between the dichroic and the camera tube lens to make sure the bottom camera is centered where the light sheet is being generated. That way objects of interest can be identified with the inverted microscope and then immediately imaged with the light sheet.
Fine Light Sheet Alignment
If the coverslip bottom is relatively small (e.g. a Matek 35mm dish) then you make sure you are imaging near the left edge of the coverslip to leave maximum room for the objective to dip below the plastic bottom.
The hardest step is finding the pencil beam with the upper microscope. There are 3 position adjustments: the SPIM head height, the SPIM head horizontal, and the manual linear positioner above the objective piezo for moving front to back as you view the microscope. Use these to move the upper objective to the right neighborhood judging by eye. The objective should be almost touching the coverslip. You can judge front to back (adjusted with the manual linear positioner) pretty well by eye. The hardest one for me is the horizontal adjustment.
Once you think you are in the right neighborhood turn on the main (top) imaging camera. Move around until you can see a line. It will start out very blurry. You can verify that you have found the beam by moving the manual linear positioner and see if the beam moves. Rotate the SPIM imaging camera so that the beam is horizontal (later you will fine tune the camera rotation). Once you have found it, move the SPIM head height and horizontal to go down near the coverslip. You can move along the beam by adjusting the SPIM horizontal by a factor of -0.5 relative to the SPIM head height movement due to the 60 degree geometry (e.g. set the increment of the height to 20um and the increment of the horizontal to -10um in the Navigation panel of the plugin and then click one-to-one.)
Use the mirror tilt of the top cube and/or the front to back knob just below to center the beam in the FOV (there is no good way of knowing which one is “right” and it doesn't matter much anyway).
Now make sure that the beam is tilted correctly. You can do this by seeing if you move in and out of focus uniformly when you adjust the piezo, understanding that the beam (and hence sheet) is diverging as it goes into solution. Adjust the beam tilt with the dichroic of the inverted microscope. It may help to stop the iris down so that there is less of a beam waist for this. You can probably get it pretty close in dye, in the long term you also want to do this with beads in solution.
Next verify that the beam waist is right near the coverslip. The way I suggest is first make sure the iris on the scanner is relatively wide open (wider beam on the edges, narrower waist). Then make sure the bottom objective is focused on the top of the coverslip using the bottom camera with transillimunation (a flashlight or even room lights from above work well). It is convenient to set this position of the lower Z axis to 0 so you can easily return. Then switch to the SPIM camera view and on the navigation tab move the lower Z negative, so the objective is focused into the solution. You should see the beam waist move into solution. The beam waist should be very near the coverslip, if not the collimator of the scanner needs adjusting (see below).
After this it is time to put beads in solution (or dip a Zebra Mildliner highlighter pen in water as shown in the diSPIM alignment video; this seems to create a suspension of small dye droplets in water). Check the beam tilt. Also check the rotation of the scanner. Similar to the diSPIM, the sheet should be uniform across the field of view and if it isn't then the scanner needs to be rotated slightly.
These adjustments get the plane of the swept beam exactly in the imaging plane, in both axes. Usually the SPIM head can be used to move into focus so you don't need the objective piezo for that, but you will need to do the 2 point calibration in the setup tab to set the slope of the piezo/light sheet calibration so that the light sheet will move with the the piezo correctly. If the slope is negative then you need to rotate the scanner 180 degrees. Verify that the slope is correct by making a “manual stack” while in live mode with the up/down arrows on the Setup tab and making sure the sample (or beads) stay in focus.
Verify that the beam waist is positioned along the optical axis as you desire (usually at the coverslip or just above because that's where your sample will be). Do not make this adjustment until everything else is aligned. Make sure that the bottom objective is focused on the coverslip surface (the sample surface, not the bottom of the coverslip!) using the inverted microscope. Next switch to the light sheet microscope and turn on the beam with the scanner iris all the way open and observe whether the beam waist is right at the coverslip. To see the beam waist it may help to move the bottom objective slightly up by a known amount (e.g. 10um) so that the beam waist is in solution instead of at the surface. Use the collimator tool to adjust the collimator until the beam waist position is correct. Only a small adjustment from factory position should be necessary.
On non-RAMM Inverted Microscope
The oSPIM was originally developed on ASI's modular inverted microscope and this is the recommended configuration. The two hurdles to overcome in putting the oSPIM on another inverted microscope are
- mounting of the oSPIM head above the sample/microscope
- getting the scanner correctly positioned to form the light sheet through the inverted microscope including
- 4F spacing (tube lens focused at scanner c-mount on one side and objective BFP on the other)
- get the beam coming into the BFP at the correct position (essential) and angle (some wiggle room)
ASI is designing mounting hardware to overcome the first hurdle as of June 2017; it will leverage the same adapter bracketry as used for the diSPIM. Overcoming the second hurdle will be up to the end user.